Tag debug info: client: {"assets":{},"datasets":{},"live":{},"projects":{},"users":{},"observable":{"assets":{},"datasets":{},"live":{},"projects":{},"users":{}}} Now: 1770625562635 Cache Key: cqdTagPageBySlug:dyslipidemia fetchCache[cqdTagPageBySlug:dyslipidemia].expirationTime: falsey fetchCache[cqdTagPageBySlug:dyslipidemia]. seconds remaining: falsey All fetchCache expiration times: -- Key: cqdNotFoundPage, seconds remaining: 1404 -- Key: cqdTagPageBySlug:artificial-heart, seconds remaining: -61 -- Key: cqdPostsByTag:cqd-migrated-tag-2945,1,10, seconds remaining: -16 -- Key: cqdTagPageBySlug:temporal-lobe-epilepsy-tle, seconds remaining: -8889 -- Key: cqdPostsByTag:cqd-migrated-tag-26494,1,10, seconds remaining: -8842 -- Key: cqdTagPageBySlug:allegra, seconds remaining: -7693 -- Key: cqdPostsByTag:cqd-migrated-tag-26001,1,10, seconds remaining: -7638 -- Key: cqdTagPageBySlug:heterotaxy, seconds remaining: -4753 -- Key: cqdPostsByTag:cqd-migrated-tag-19967,1,10, seconds remaining: -4704 -- Key: cqdTagPageBySlug:machine-learning, seconds remaining: -4041 -- Key: cqdPostsByTag:cqd-migrated-tag-18105,3,10, seconds remaining: -3994 -- Key: cqdTagPageBySlug:lung-parenchyma, seconds remaining: -1455 -- Key: cqdPostsByTag:cqd-migrated-tag-25828,1,10, seconds remaining: -1403 -- Key: cqdTagPageBySlug:nurse-satisfaction, seconds remaining: -1354 -- Key: cqdPostsByTag:cqd-migrated-tag-17687,1,10, seconds remaining: -1311 -- Key: cqdTagPageBySlug:nuss-procedure, seconds remaining: -404 -- Key: cqdPostsByTag:cqd-migrated-tag-19966,1,10, seconds remaining: -355 -- Key: cqdTagPageBySlug:ozaki-procedure, seconds remaining: 359 -- Key: cqdPostsByTag:cqd-migrated-tag-24932,1,10, seconds remaining: 403 -- Key: cqdTagPageBySlug:dietary-supplements, seconds remaining: 1404 -- Key: cqdPostsByTag:cqd-migrated-tag-18776,1,10, seconds remaining: 1451 -- Key: cqdTagPageBySlug:beth-meese, seconds remaining: 1630 -- Key: cqdPostsByTag:cqd-migrated-tag-1425,1,10, seconds remaining: 1674 -- Key: cqdTagPageBySlug:online-courses, seconds remaining: 1971 -- Key: cqdPostsByTag:cqd-migrated-tag-17824,1,10, seconds remaining: 2013 -- Key: cqdTagPageBySlug:aaos-2019, seconds remaining: 2080 -- Key: cqdPostsByTag:cqd-migrated-tag-20262,1,10, seconds remaining: 2132 -- Key: cqdTagPageBySlug:arthroplasty, seconds remaining: 2192 -- Key: cqdPostsByTag:cqd-migrated-tag-23652,1,10, seconds remaining: 2239 -- Key: cqdTagPageBySlug:pre-exposure-prophylaxis, seconds remaining: 2319 -- Key: cqdPostsByTag:cqd-migrated-tag-19947,1,10, seconds remaining: 2357 -- Key: cqdTagPageBySlug:asco-2020, seconds remaining: 3072 -- Key: cqdPostsByTag:cqd-migrated-tag-22259,1,10, seconds remaining: 3119 -- Key: cqdTagPageBySlug:humeral-component, seconds remaining: 3750 -- Key: cqdPostsByTag:cqd-migrated-tag-21372,1,10, seconds remaining: 3795 -- Key: cqdTagPageBySlug:study, seconds remaining: 4468 -- Key: cqdPostsByTag:cqd-migrated-tag-2148,2,10, seconds remaining: 4510 -- Key: cqdTagPageBySlug:distress-screening, seconds remaining: 5231 -- Key: cqdPostsByTag:cqd-migrated-tag-1132,1,10, seconds remaining: 5278 -- Key: cqdTagPageBySlug:overdose-risk-score, seconds remaining: 7961 -- Key: cqdPostsByTag:cqd-migrated-tag-24973,1,10, seconds remaining: 8006 -- Key: cqdTagPageBySlug:osteobesity, seconds remaining: 8646 -- Key: cqdPostsByTag:cqd-migrated-tag-25271,1,10, seconds remaining: 8698 -- Key: cqdTagPageBySlug:oncotype-dx, seconds remaining: 8891 -- Key: cqdPostsByTag:cqd-migrated-tag-21536,1,10, seconds remaining: 8941 -- Key: cqdTagPageBySlug:emergency-nurses, seconds remaining: 9142 -- Key: cqdPostsByTag:cqd-migrated-tag-18518,1,10, seconds remaining: 9217 -- Key: cqdTagPageBySlug:intraoperative-oct, seconds remaining: 9313 -- Key: cqdPostsByTag:cqd-migrated-tag-380,1,10, seconds remaining: 9360 conditions: -- false, -- NA, -- NA, -- NA -- false Cache miss for key cqdTagPageBySlug:dyslipidemia - retrieving from Sanity CCCache.dataFetchCount: 25720 Cache cleanup seconds remaining: 11111
Advertisement
Advertisement
First-in-human phase 1 trial induced loss of function in gene that codes for ANGPTL3
Yet 21.4% of tested individuals had Lp(a) elevation
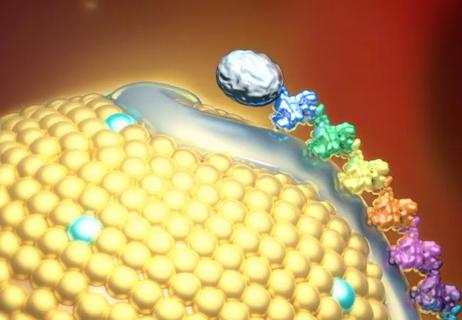
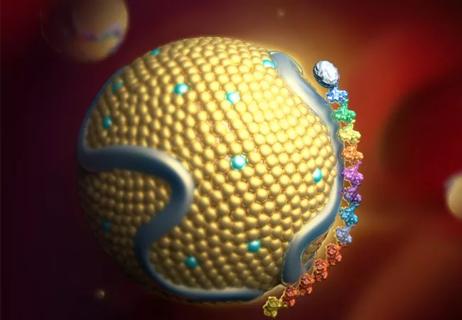
Small interfering RNA lowered lipoprotein(a) by 94% during six-month follow-up after a single dose
Tech-assisted self-selection concurred with clinician-assessed eligibility in >90% of cases
Advertisement
Cleveland Clinic is a non-profit academic medical center. Advertising on our site helps support our mission. We do not endorse non-Cleveland Clinic products or services. Policy
Newly identified pathway may explain the so-called niacin paradox
It's time to increase testing for this major cardiovascular risk factor in advance of new therapies
Undetectable levels achieved for nearly nine months in phase 1 trial
Randomized controlled study undercuts unsubstantiated ‘heart health’ claims
Targeted mRNA blocking could be first effective approach specific to this cardiovascular risk factor
Preclinical models focus interest on the protein hepsin
Rendered: Mon Feb 09 2026 08:26:02 GMT+0000 (Coordinated Universal Time)
9500 Euclid Avenue, Cleveland, Ohio 44195 |
800.223.2273 | ©
2026 Cleveland Clinic. All Rights Reserved.