First in-human trial using CRISPR/CASP 12 for gene editing in sickle cell disease
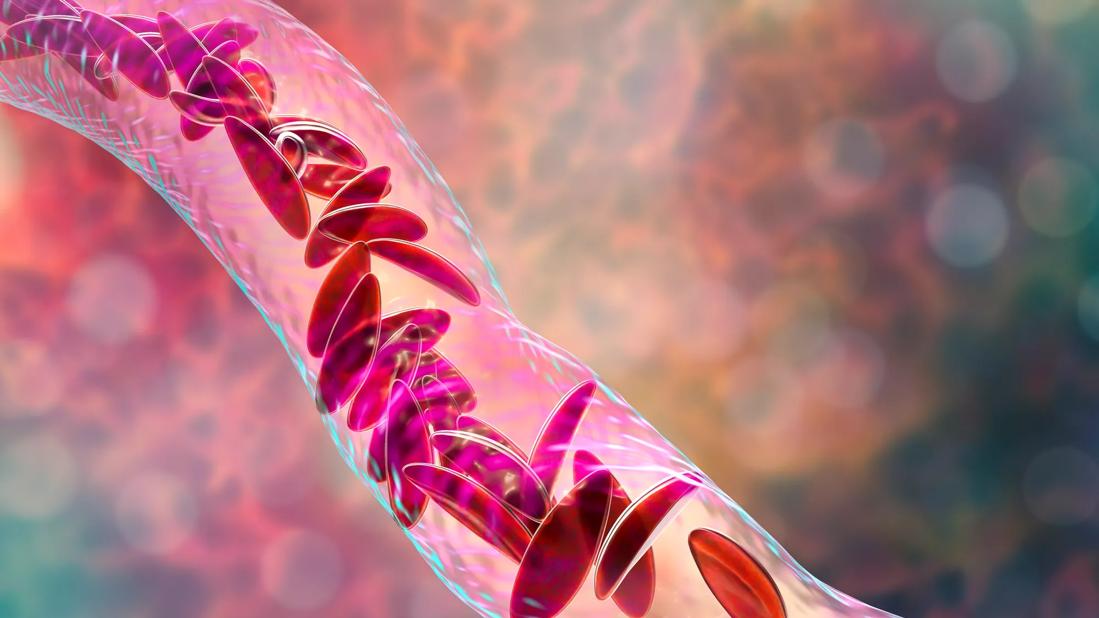
A long-awaited curative treatment for sickle cell disease has moved closer to reality: the preliminary results of a clinical trial of a gene therapy show that it is safe and effective. Cleveland Clinic is participating in the multicenter Phase I/II trial, RUBY, which is evaluating EDIT-301, an experimental one-time gene editing cell therapy.
Advertisement
Cleveland Clinic is a non-profit academic medical center. Advertising on our site helps support our mission. We do not endorse non-Cleveland Clinic products or services. Policy
Sickle cell disease is a hereditary blood disorder caused by a single mutation in the hemoglobin gene. The genetic change in DNA leads to a polymerization of the sickle hemoglobin (HbS), which causes red blood cells to form a sickle shape instead of a normal disc shape. These sickle cells have a shortened lifespan and block blood flow which causes anemia, pain crises, organ failure and early death. Patients with higher levels of fetal hemoglobin (HbF) inhibit HbS polymerization, which reduces sickle formation.
For the estimated 100,000 people in the United States currently living with sickle cell disease, medications can modify disease severity and treat symptoms, but lifespan is still in the mid-40s, which is almost 30 to 40 years below life expectancy for normal U.S. populations. The only curative treatment is an allogenic blood or marrow transplant, but it is very difficult to find a suitable match. The treatment also poses a risk of rejection in addition to graft versus host disease, which necessitates the need for immune suppression.
“We have made progress with treatments that modify the disease and reduce pain and the need for hospital admissions, but there is no treatment that can address the disease itself except for BMT,” says Rabi Hanna, MD, Chairman of the Department of Pediatric Hematology, Oncology and Bone Marrow Transplant at Cleveland Clinic.
Sickle cell disease has long been considered a prime candidate for gene therapy because it involves a single genetic mutation, but it is only recently that the technology has become accurate and precise enough to be effective.
Advertisement
EDIT-301 uses a novel type of CRISPR gene editing technology – known as CRISPR/CASP 12. It has produced genomic modification that mimics naturally occurring mutations of hereditary persistence of fetal hemoglobin (HPFH) in the γ-globin gene (HBG1 and HBG2) promoters to reactivate γ-globin expression and increase HbF production. These patients have a very mild phenotype and do not seem to be affected by regular complications of pain and end-organ damage that occurs in other sickle cell patients.
First, the patient’s hematopoietic stem cells are harvested from peripheral blood after they are given a medication called plerixafor. These cells are sent to EDITAS Medicine, a genome editing company, where they edit the promoter regions of gamma globin gene 1 and 2 using CRISPR/CASP 12 to increase HbF production, which mimics the natural mechanism of HbF to modify the disease process.
Patients are then treated with chemotherapy to make room for the genetically modified cells, which are delivered by infusion—similar to any blood transfusion. Patients remain in the hospital until they engraft with the new modified stem cells to produce the edited healthy red blood cells.
“CRISPR/CASP 12 is an incredibly precise technology that goes directly toward the defect or the targeted gene and cuts it out, so it can be replaced with a gene to activate HbF production. It induces changes in the HBG1 and HBG2 promoters to reactivate γ-globin expression and increases HbF production similar to what is seen in naturally occurring HPFH,” explains Dr. Hanna.
Advertisement
Dr. Hanna and a group of clinicians from institutions participating in the trial analyzed the preliminary data from two patients who received EDIT-301 treatment.
The study is open to patients ages 18 to 50 with a diagnosis of severe sickle cell disease, which is defined as two or more vaso-occlusive events (VOEs) per year in the two-year period prior to enrolling in the trial.
At the time of the study, March 2023, the two participants were eight and four months post-EDIT-301 treatment, respectively. Neutrophil and platelet engraftment were achieved within 23 and 19 days (participant one) and 29 and 37 days (participant two). Both had favorable outcomes: successful engraftment, a rapid and sustained increase in total Hb and HbF levels and a higher percentage of F-cells, and improvements in key markers of hemolysis. No VOEs were reported. The treatment was safe with no treatment-related adverse events and no serious adverse events.
“These results are very encouraging. Our hope is to achieve a potentially functional cure so that the disease won’t affect the patient’s daily life. It wouldn’t change the impact of sickle cells that are already present in the body, but it would help prevent any future organ damage caused by sickle cells,” says Dr. Hanna.
The study aims to enroll 20 patients this year; six have been enrolled, and four have received treatment. Patients will be monitored closely after treatment for up to two years. Once safety and efficacy are demonstrated in adults, trials could be conducted in pediatric sickle cell disease patients.
Advertisement
“We need more long-term data to be confident that we can safely and effectively cure sickle cell disease,” says Dr. Hanna. “Our hope is that in the coming year, there will be a gene therapy available that can save patients from this life-threatening disease.”
Advertisement
Advertisement

Interim results of RUBY study also indicate improved physical function and quality of life

Integrated care model reduces length of stay, improves outpatient pain management

New course offers insights into clinical, psychosocial and ethical dimensions of care

Watch for sudden unilateral vision loss without pain

Efficacy, safety and tolerability data shared at hematology meeting
Nurses play key role in comprehensive lifetime treatment program

How to combat the rise in mortality when patients become adults

Two-year event-free survival comparable to matched sibling donor myeloablative transplant