Ideal protocols feature frequent monitoring, high-quality imaging and a team approach
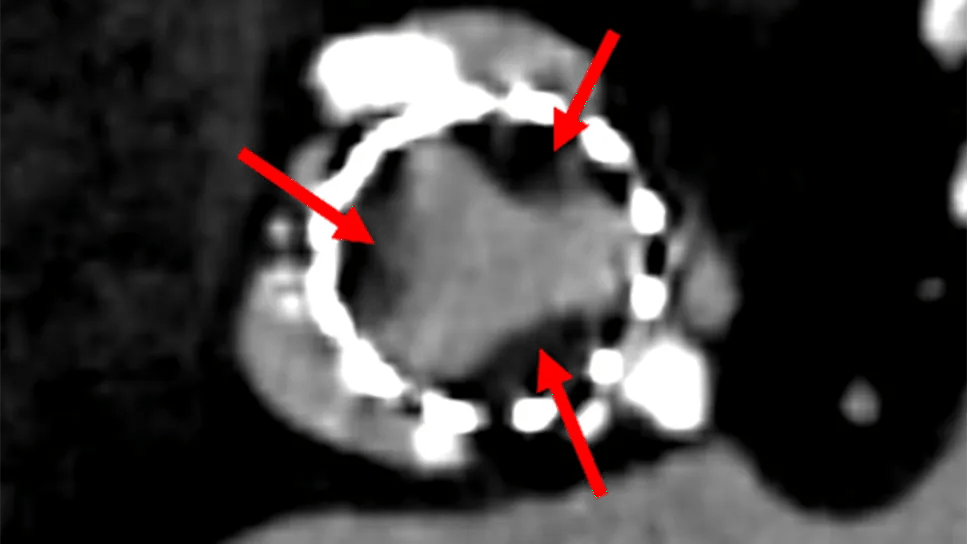
Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/56c39ae9-378b-4ef7-bfc9-5295cbad639c/aortic-valve-replacement-feature)
bioprosthetic heart valve with thrombosis seen on imaging study
Frequent monitoring to enable early identification and treatment of potential abnormalities is key to the long-term success of aortic valve replacement (AVR), whether surgical (SAVR) or transcatheter (TAVR), say Cleveland Clinic heart valve specialists.
Advertisement
Cleveland Clinic is a non-profit academic medical center. Advertising on our site helps support our mission. We do not endorse non-Cleveland Clinic products or services. Policy
“As a large referral center for valvular heart disease, we see a substantial number of patients who develop valvular stenosis, valvular regurgitation or paravalvular regurgitation soon after SAVR or TAVR,” says Amar Krishnaswamy, MD, Section Head of Invasive and Interventional Cardiology. “If any of these conditions is left unaddressed until the patient develops symptoms that would prompt testing, it could result in irrecoverable cardiac damage.”
Because the risks of early complications are similar in patients undergoing TAVR or SAVR, the follow-up protocol at Cleveland Clinic is essentially the same for both.
Echocardiography is the primary modality used for routine follow-up. It allows for visualization of the valve and assessment of blood flow through the valve.
The first echocardiogram is performed during SAVR or TAVR. A second echocardiogram may be performed on the day of or day following TAVR or two to three days after SAVR; this second echo serves as the patient’s new “baseline” study.
A third echocardiogram is performed in the two- to three-month follow-up window. Its primary purpose is to identify any increase in valve gradients or development of valvular regurgitation — and, if either is present, to determine the location and severity.
“Often these problems are small, but sometimes they are more than mild,” notes L. Leonardo Rodriguez, MD, a staff cardiologist in the Section of Cardiovascular Imaging. “However, they are easily detected early on and easy to follow over time.”
Advertisement
“Changes in gradients can be somewhat subtle, even remaining in the normal range,” Dr. Krishnaswamy adds. “For tissue valves, an early increase in gradients could be associated with thrombosis. We compare this two- to three-month follow-up study to the postprocedure baseline study to see whether we are prompted to look further with CT imaging.”
If a tissue valve appears abnormal on echocardiography during the first few months or couple of years after implantation, a CT scan may be obtained. CT is the gold standard for diagnosing premature leaflet calcification and hypoattenuated leaflet thickening/thrombosis (HALT) (Figure 1), both of which can impair valve function.
“With a high-quality CT scan, you can see a thin film of thrombosis on the base of the leaflet,” Dr. Rodriguez notes. “Sometimes you can actually visualize restricted motion of the leaflets.”
“Whether or not HALT should be treated is decided on a case-by-case basis and may entail the use of anticoagulants,” says Dr. Krishnaswamy. “When patients are not good candidates for anticoagulation, we may choose to observe them and repeat the echo a few months later. In half of patients, HALT resolves spontaneously. If their next echo is normal, we then recommend monitoring with annual echocardiography.” What is not known is whether HALT may cause stroke or later calcification of the valve leaflets.

Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/d6991eef-f8b3-45ff-b333-2d52ad77fdf6/aortic-valve-replacement-scan1)
Figure 1. Hypoattenuated leaflet thickening/thrombosis (HALT) of a TAVR valve. CT image (left panel) shows thrombus (arrows) on the leaflets with resulting high echocardiographic gradients (middle panel) that resolved after anticoagulation (right panel).
For patients who present with fever or other symptoms suggestive of endocarditis, or when new leakage of the valve is detected that could be “paravalvular” (Figure 2), a transesophageal echocardiogram may be necessary. Paravalvular leakage is usually due to failure of one or more sutures holding the valve in place or to incomplete TAVR valve deployment.
Advertisement

Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/4d779f74-772b-481a-ba6f-5160461df7f1/aortic-valve-replacement-scan2)
Figure 2. Paravalvular leak of a SAVR valve and its subsequent treatment. Space (arrowhead in left panel) between the valve (arrow in left panel) and the cardiac tissue is filled using special plug devices (arrow in right panel) via a catheter-based procedure.
After TAVR or SAVR, Cleveland Clinic valve specialists follow patients with yearly echocardiograms.
“It’s important to maintain vigilant surveillance, because it’s likely that younger patients may outlive their current valve and require another valve replacement,” Dr. Krishnaswamy says.
“Changes in tissue valves are often subtle and gradual, but a subset of patients experience rapid deterioration for unknown reasons,” Dr. Rodriguez adds. “It’s good to know the trajectory of these changes early on. Follow-up of mechanical valves is recommended based on symptoms. However, these valves also may develop complications over time. That is why it is so helpful to have a history of imaging.”
At Cleveland Clinic, the care of patients with valvular heart disease is handled by a multidisciplinary team of experts.
“We have a number of caregivers in different specialties who collaborate in the management of these patients,” says Marijan Koprivanac, MD, a cardiothoracic surgeon whose specialty interests include aortic valve disease. “We surgeons care for SAVR patients while they are in the hospital, but they are followed longitudinally by a cardiologist, who will call on us if needed.”
The patient’s local provider is also included in the care team. “We never see the care we provide as exclusionary,” Dr. Krishnaswamy says. “We do our best to communicate our findings and our treatments to the referring provider. We like to see our patients follow up with referring physicians 7 to 10 days after discharge, and our nurse practitioners schedule their appointments. We are all one team.”
Advertisement
Many patients who live locally return to Cleveland Clinic yearly for follow-up. When travel isn’t feasible, telephone or video visits may be arranged.
When patients have complex or unresolved issues, arrangements may be made for them to alternate every six months between seeing their Cleveland Clinic cardiologist and seeing their local provider.
The role and extent of local follow-up is determined in large part by the availability of quality echocardiography studies. “The quality of imaging is paramount, as some of the valve abnormalities are important if they are detected or missed,” Dr. Rodriguez explains.
CT scanning is generally less variable, but a scanner without cardiac gated software will be suboptimal.
Cleveland Clinic’s electronic medical record system prompts physicians to schedule patients for a return visit one year after their current visit and to undergo necessary testing if desired. This minimizes chances of patients being lost to follow-up.
Nevertheless, it is incumbent on patients to keep these appointments with their local provider or at Cleveland Clinic. “We rely on patients to be aware of what their follow-up should be,” Dr. Krishnaswamy notes. “This is why it’s important for referring physicians to have conversations with their patients about the need for appropriate follow-up. Our team approach with local physicians extends to the patient as well.”
Advertisement
Advertisement
Modified-Bentall single-patch Konno enlargement (BeSPoKE) optimizes hemodynamics, facilitates future TAVR
Experience-based takes on valve-sparing root replacement from two expert surgeons
30-year study of Cleveland Clinic experience shows clear improvement from year 2000 onward
Surgeons credit good outcomes to experience with complex cases and team approach
For many patients, repair is feasible, durable and preferred over replacement
In experienced hands, up to 95% of patients can be free of reoperation at 15 years
Experience and strength in both SAVR and TAVR make for the best patient options and outcomes
20 years of Cleveland Clinic experience in ~500 patients with proximal aortic aneurysm or dilation