Novel approach combines magnetic hyperthermia, amino acid gel to disrupt bacterial biofilm
By Anabelle Visperas, PhD; Alison Klika, MS; Ana Cristina Samia, PhD; Carlos A Higuera-Rueda, MD
Advertisement
Cleveland Clinic is a non-profit academic medical center. Advertising on our site helps support our mission. We do not endorse non-Cleveland Clinic products or services. Policy
Infection after total joint arthroplasty is a devastating complication made exceptionally problematic due to the difficulties in diagnosing and treating such infections. The current options to treat chronic infections, including one-stage or two-stage revision surgeries, do not always eradicate these infections and are relatively high in morbidity.
There is a need for innovative methods for biofilm disruption and infection eradication, which may optimize the effects of antibiotics or even potentially offer an alternative to antibiotic strategies. If the biofilm and bacteria can be suppressed or eradicated without the use of extensive surgery and potentially preserve the prosthesis, then the devastating effects of periprosthetic joint infection (PJI) can be mitigated.
In a joint effort with colleagues at Case Western Reserve University, Cleveland, we have developed an innovative approach to treating PJI that combines magnetic hyperthermia and amino acid gel. It holds promise to address this confounding problem and is now in preclinical testing.
Bacterial biofilms are antibiotic-resistant cell associations that have been estimated to be involved in up to 80 percent of infections of the body, and are commonly associated with growth on foreign bodies such as prosthetic joint implants. Their impenetrable nature presents a challenge to successful eradication, as bacteria associated with biofilms can be physically protected from antibiotic treatment and host defenses. Also, due to the structure of biofilm, bacteria may live in a metabolically inert state, making antibiotic use suboptimal. Whereas planktonic bacteria can be collected and cultured more readily, bacteria incorporated within a biofilm may be hard to sample for identification and optimal treatment.
Advertisement
Recently, basic science research laboratories have developed many promising antibacterial methods and solutions to ablate bacterial biofilm. While some methods have had encouraging results in vitro, they have yet to be proven in the clinical setting.
Magnetic hyperthermia uses nanoparticles that generate localized heat upon exposure to an alternating current (AC) magnetic field. This treatment modifies bacterial cell surface and biofilm structure, independent of antibacterial use, making it an attractive option for antibiotic-resistant strains.
At the same time, amino acids have been reported to interfere with metabolic pathways of bacteria, thus disrupting biofilm activity. Indeed, various D-amino acids (D-AAs, D-tyr, D-trp, and D-phe) have shown maximum efficacy in biofilm disruption, in contrast to vancomycin treatment which has shown minimal Staphylococcus aureus (S. aureus) biofilm disruption at clinically therapeutic doses.
The high concentration of D-AAs required is toxic to mammalian cells when exposed for long periods of time. Time-dependent HeLa cell viability assays identified two hours as the optimal exposure time at which the D-AAs can adequately disrupt the biofilm (85 percent disruption) with minimal cell damage.
The combination of magnetic hyperthermia activation with D-AA treatment offers an unexplored treatment option to combat biofilm.
In collaboration with Case Western Reserve University, we have developed a novel combination of antibacterial D-AA and a thermoresponsive magnetic glycol-based nanocomposite with iron oxide nanoparticles (MagDAA gel) that works synergistically to eradicate S. aureus biofilm.
Advertisement
This 5 percent glycol-based chitin solution (hydrogel) containing c-MNPs (cubic magnetic nanoparticles) was optimized to be in a liquid state when cool and gel state at body temperature for maximal targeting to infected tissue. Treatment with the MagDAA gel solution alone did not completely disrupt biofilm. However, treatment with a magnetic field to incubated MagDAA gel led to complete biofilm eradication in contrast to antibiotics solutions (Figure 1).
The next step on this investigation journey will assess the efficacy and safety of this solution to eradicate bacterial biofilm in an in vivo rabbit model of PJI. Rabbits will receive a tibial implant, manufactured by the Cleveland Clinic Biomedical Engineering Department, and infected with S. aureus. After a productive infection has been confirmed, the D-AA/hyperthermia nanocomposite gel solution will be tested for biofilm disruption and potential eradication of infection.
This can potentially set the basis to translate this solution into clinical practice.
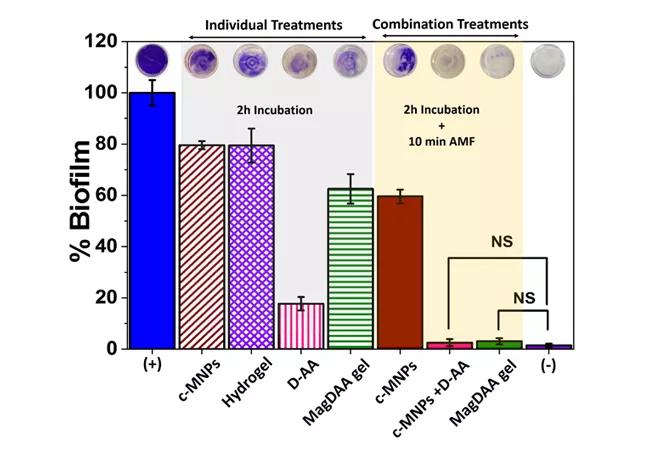
Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/7de840cf-8725-4311-afbb-dbd7f62e52dd/18-ORT-1197-Higuera-Inset-Image-650x450_jpg)
Figure 1. MagDAA gel has maximal biofilm disruption following magnetic field (AMF) treatment. S. aureus was cultured for 24 hours for biofilm formation. Media was removed and biofilm was incubated with separate components of MagDAA gel – c-MNPs (cubic magnetic nanoparticles), hydrogel, D-AA – and the combination of all components. Maximal biofilm disruption was attained with combination treatment of two-hour MagDAA incubation followed by AMF treatment for 10 minutes at AC field amplitude of 5kA/m and a frequency of 380 kHz, using crystal violet staining biofilm disruption assay. NS indicates that the difference in means of the treatments are not statistically significant at P > 0.05.
Advertisement
About the authors: Dr. Visperas is a Research Coordinator in the Department of Orthopaedic Surgery; Alison Klika is a Research Program Manager in the Department of Orthopaedic Surgery; Dr. Samia is Associate Professor, Department of Chemistry, CWRU; and Dr. Higuera-Rueda is Chairman, Department of Orthopaedic Surgery, Cleveland Clinic Florida.
Suggested reading
Abenojar EC, et al. Magnetic glycol chitin-based hydrogel nanocomposite for combined thermal and d-amino-acid assisted biofilm disruption. ACS Infect Dis. 2018;4(8):1246-1256.
McConoughey SJ, et al. Biofilms in periprosthetic orthopedic infections. Future Microbiol. 2014;9(8):987-1007
Parvizi J, Gehrke T. Definition of periprosthetic joint infection. J Arthroplasty. 2014;29(7):1331
Advertisement
Advertisement

How it’s similar but different from the direct anterior approach

Collaboration must cross borders and disciplines

Systematic review of MOON cohorts demonstrates a need for sex-specific rehab protocols

Should surgeons forgo posterior and lateral approaches?

How chiropractors can reduce unnecessary imaging, lower costs and ease the burden on primary care clinicians

Why shifting away from delayed repairs in high-risk athletes could prevent long-term instability and improve outcomes

Multidisciplinary care can make arthroplasty a safe option even for patients with low ejection fraction

Percutaneous stabilization can increase mobility without disrupting cancer treatment