An abundance of promising studies are on the horizon
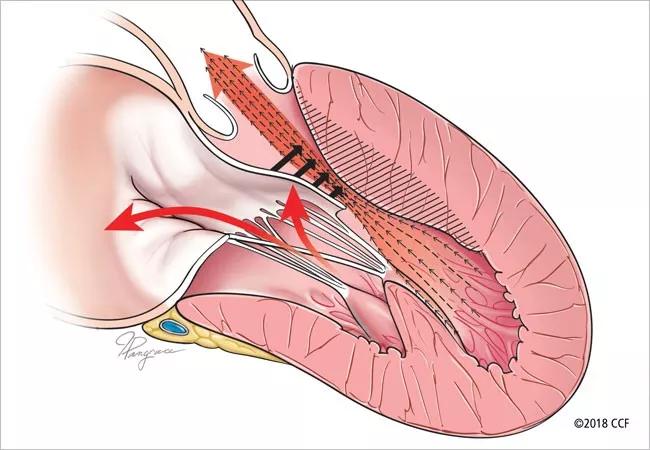
When Cleveland Clinic cardiologist Milind Desai, MD, MBA, presented findings from the pivotal VALOR-HCM study of the cardiac myosin inhibitor mavacamten at last year’s American College of Cardiology and American Heart Association meetings, it was just the start of a wave of emerging data from hypertrophic cardiomyopathy (HCM) investigations.
Advertisement
Cleveland Clinic is a non-profit academic medical center. Advertising on our site helps support our mission. We do not endorse non-Cleveland Clinic products or services. Policy
In addition to VALOR-HCM substudies and long-term follow-up of the trial’s enrollees, reports are anticipated from a registry of real-world use of mavacamten, a Cleveland Clinic-led trial of mavacamten for nonobstructive HCM, a phase 3 study of a second cardiac myosin inhibitor for obstructive HCM and early studies of a novel gene therapy for MYBPC3-associated nonobstructive HCM. These come on the heels of a newly published Medicare database analysis with implications for the use of new drug therapies in the mix of therapeutic options for HCM. Cleveland Clinic has a role in all these investigations, which are outlined below.
Mavacamten’s FDA approval last spring as the first medication for treatment of symptomatic obstructive HCM was based largely on VALOR-HCM, which randomized 112 patients with symptomatic obstructive disease to mavacamten or placebo. Through the latest follow-up, at 32 weeks, mavacamten significantly reduced patients’ eligibility for septal reduction therapy (SRT) relative to placebo, the study’s primary endpoint, as detailed in an earlier Consult QD post.
Follow-up of the study’s participants will continue through at least 128 weeks, says Dr. Desai, the study’s national principal investigator and Director of the Hypertrophic Cardiomyopathy Center at Cleveland Clinic. He notes that one-year data will be available later this year. “We are focused on confirming whether the impressive safety and efficacy results with mavacamten are sustainable over the long term,” he says.
Advertisement
“Now that we have evidence of symptom improvement with mavacamten over eight months, we are eager to learn whether its long-term use reduces life-threatening arrhythmias and sudden death,” adds Nicholas Smedira, MD, MBA, Surgical Director of Cleveland Clinic’s Hypertrophic Cardiomyopathy Center. “An effective noninvasive alternative to septal reduction therapy would provide a much-needed option for highly symptomatic patients and expand our toolbox of offerings.”
Meanwhile, VALOR-HCM substudies are emerging. One led by Cleveland Clinic cardiologist Paul Cremer, MD, MS, showed that mavacamten improved measures of diastolic function independent of its effects in reducing left ventricular outflow tract gradients and mitral regurgitation (Circ Cardiovasc Imag. Epub 6 Nov 2022). “This has important implications for the use of diastolic assessment to determine prognosis and evaluate treatments,” says Dr. Cremer. Another substudy will soon be presented on mavacamten’s effects on Kansas City Cardiomyopathy Questionnaire results.
Additional insights on mavacamten will be coming from DISCOVER-HCM (NCT05489705), a prospective U.S. registry study of the drug’s real-world use that launched in late 2022. “From this we will learn things like how many patients receiving mavacamten have declines in ejection fraction and how many continue to feel good symptomatically over time,” Dr. Desai notes.
He also will be watching whether mavacamten’s effects are ultimately shown to extend beyond symptoms, diastolic function and avoidance of SRT to include structural changes in the heart, such as regression of mass. “A key question is whether this can modify the disease to a point where we can change patients’ life trajectory in a positive way,” he says.
Advertisement
Meanwhile, an administrative database study by Dr. Desai and Cleveland Clinic colleagues (J Am Coll Cardiol. 2023;81:105-115) suggests a possible role that drugs like mavacamten might play in the future therapeutic landscape for obstructive HCM. The researchers analyzed more than 5,000 Medicare beneficiaries who underwent SRT — i.e., septal myectomy or alcohol septal ablation — from 2013 through 2019. They found that while both forms of SRT significantly reduced readmissions for heart failure, septal myectomy was associated with lower redo rates and better long-term survival relative to alcohol septal ablation.
Notably, the analysis also showed that despite better SRT outcomes at high-volume centers, 70% of SRT procedures in the U.S. were performed at low-volume centers. “Despite guideline recommendations, many patients are still undergoing SRT at low-volume centers,” Dr. Desai observes. He says this suggests particular potential utility for cardiac myosin inhibitor therapy in patients who cannot easily access a high-volume SRT center, “given that outcomes are inferior at low-volume centers.”
Such patients may ultimately benefit from additional choice in cardiac myosin inhibitor therapy, as a second drug in the class — aficamten — is now in phase 3 testing for symptomatic obstructive HCM. The SEQUOIA-HCM study (NCT05186818), in which Cleveland Clinic is a participating center, is expected to be completed later this year.
Additionally, Cleveland Clinic is leading a phase 3 multicenter trial of mavacamten in a different patient population — those with nonobstructive HCM. ODYSSEY-HCM (NCT05582395) is a randomized, double-blind, placebo-controlled study run by the Cleveland Clinic Coordinating Center for Clinical Research (C5Research). The trial is enrolling several hundred patients with symptomatic nonobstructive HCM for treatment over one year to evaluate effects on quality of life, functional capacity and biomarkers.
Advertisement
If the results are positive, mavacamten could become the first medical therapy approved for nonobstructive HCM, which rarely is treated with SRT. “Patients with nonobstructive disease are more challenging to treat,” says Dr. Desai, who is chair of the ODYSSEY-HCM executive committee. “We are looking to see if cardiac myosin inhibition improves functional quality of life in a setting where there is no obstruction to relieve.”
Cleveland Clinic is also one of a small number of centers involved in early studies of a gene therapy approach to HCM. The strategy is targeted at HCM due to MYBPC3 mutation, a protein deficiency mutation and the most prevalent genetic form of HCM.
The approach involves TN-201, an investigational adeno-associated virus-based gene therapy designed to address the underlying cause of MYBPC3-associated HCM by delivering a functional MYBPC3 gene to restore normal levels of myosin-binding protein C3. “The virus is administered as a single injection to stimulate protein formation and theoretically result in normal myocardial thickness,” Dr. Desai explains, noting that animal studies have shown successful mass regression. “If it’s ultimately shown to be safe and effective in humans, it could prove to be a one-time solution for a fairly common form of HCM.”
Advertisement
Advertisement

End-of-treatment VALOR-HCM analyses reassure on use in women, suggest disease-modifying potential

Cardiac imaging substudy is the latest paper originating from the VANISH trial

Vigilance for symptom emergence matters, a large 20-year analysis reveals

Phase 3 ODYSSEY-HCM trial of mavacamten leaves lingering questions about potential broader use

5% of flagged ECGs in real-world study were from patients with previously undiagnosed HCM

High composite score in myectomy specimens signals worse prognosis
Few patients report left ventricular dysfunction or heart failure after one year
Avoidance of septal reduction therapy continues while LVEF dysfunction remains infrequent