
Insights on ex vivo lung perfusion, dual-organ transplant, cardiac comorbidities and more

Milestone minimally invasive surgeries reduce pain and recovery time

Enhanced visualization and dexterity enable safer, more precise procedures and lead to better patient outcomes

Insights on bringing Cleveland Clinic even closer to becoming the best transplant enterprise in the world
Advertisement
Cleveland Clinic is a non-profit academic medical center. Advertising on our site helps support our mission. We do not endorse non-Cleveland Clinic products or services. Policy

Minimally invasive approach, peri- and postoperative protocols reduce risk and recovery time for these rare, magnanimous two-time donors

Minimally invasive pancreas-kidney replacement reduces patient’s pain, expedites recovery

Highlights and insights from recent Cleveland Clinic experience

First-ever procedure restores patient’s health
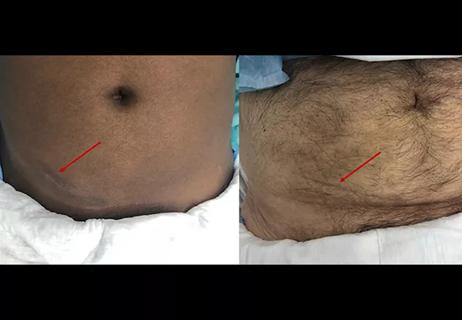
Smaller incision may lead to reduced postoperative pain for some patients

Long-term lung allograft outcomes clinically unaffected by organ exposure, study finds
Advertisement
Advertisement