Clinical trials show promise in restoring vision or stopping vision loss
By Meghan DeBenedictis, MS, CGC, MEd, and Elias I. Traboulsi, MD, MEd
Advertisement
Cleveland Clinic is a non-profit academic medical center. Advertising on our site helps support our mission. We do not endorse non-Cleveland Clinic products or services. Policy
The Center for Genetic Eye Diseases at Cole Eye Institute has seen significant growth in number of patients with inherited retinal dystrophies (IRDs) served over the last several years.
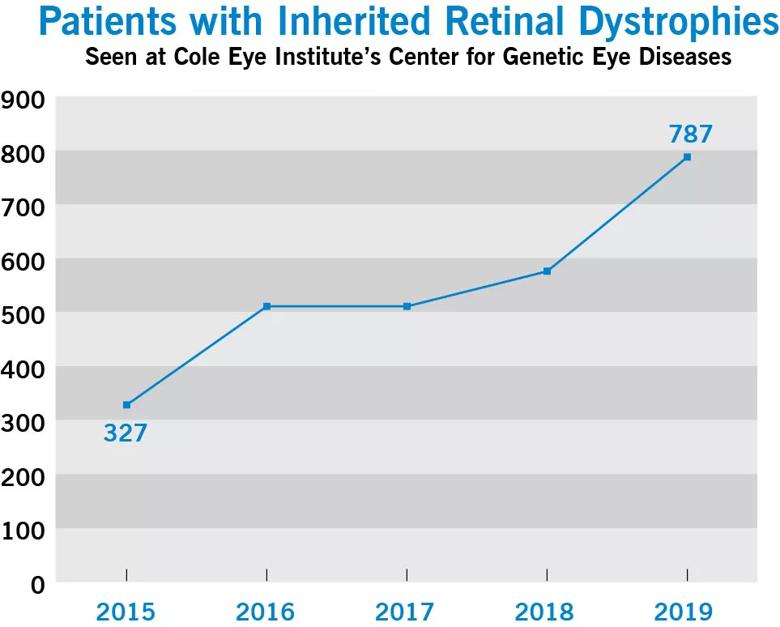
Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/e94e49fc-3ec2-4940-9310-a926eb139078/20-EYE-1875134-CQD-Gene-Therapy-Chart_jpg)
This increase is largely due to advances in the field of ophthalmic genetics, the availability of commercial and high-yield molecular genetic testing, and rising interest in clinical trial participation.
Historically, the diagnosis of rare inherited eye diseases was based on clinical examination by ophthalmologists with experience in the field, who could recognize the particular clinical manifestations and combine them with pedigree analysis and limited imaging and electrophysiologic testing.
Over the last two decades, more than 270 genes have been associated with IRDs. These IRDs can be inherited in an autosomal dominant, recessive or X-linked manner.1
While some IRDs have characteristic clinical findings, many do not, making a precise diagnosis challenging. Further complicating the picture is the presence of systemic abnormalities that, in some cases, can be serious or even life-threatening and indicate syndromic disease, requiring additional medical attention.
Molecular genetic testing has become a major tool to that effect. Advances in genetic testing technology have made molecular diagnosis possible in more than 70% of cases.2 This percentage continues to increase as knowledge of genetic variants and their contributions to disease improves and as disease-gene discovery continues.
The Center for Genetic Eye Diseases is best positioned to evaluate patients, and confirm or refine the diagnosis of IRDs using the expertise of its staff, state-of-the-art imaging and the most up-to-date array of molecular genetic testing. Expert ophthalmic genetic counseling services are provided to all patients.
Advertisement
Genetic testing should only be obtained with the input of an ophthalmologist with expertise in the field and/or a certified genetic counselor who specializes in ophthalmic genetics. These experts help ensure that the most appropriate test is ordered, results are accurately interpreted and cascade testing is pursued for family members when indicated.
Diagnostic genetic test results can incite powerful feelings in the patient and at-risk family members. Therefore, pre- and post-test counseling by a genetic counselor is highly recommended.
Ophthalmic genetic counselors also are well-positioned to work with patients and ophthalmologists to determine eligibility for clinical gene therapy trials. This has become one of the primary reasons patients seek care from the Cole Eye Institute team.
The most common type of gene therapy is gene-replacement therapy, in which the disease-causing gene is “replaced” with a healthy, working copy of the gene. Retinal gene therapy is typically administered by vitrectomy and subretinal injection of an adeno-associated viral vector containing the gene of interest, resulting in normal protein production and function.
Gene therapy has received significant attention in recent years, especially in the inherited eye disease community.
In December 2017, the U.S. Food and Drug Administration announced approval of the first gene therapy for a monogenic disease — biallelic RPE65-related retinal dystrophy. This treatment, voretigene neparvovec ophthalmic (Luxturna®), delivers a working copy of the RPE65 gene directly to the retina of affected individuals. Patients receiving this treatment, who often have profound night blindness, showed significant improvement in navigating an obstacle course at low light levels, according to one study.3
Advertisement
The success of RPE65 gene therapy has spurred an increased interest in gene therapy trials for other IRDs. Currently there are more than 30 gene therapy clinical trials being conducted in the U.S. alone. Some include:
Gene therapy continues to show promise in either restoring vision or halting progression of vision loss in patients affected by these conditions.
Having successfully participated in natural history studies, such as ProgStar for Stargardt disease, the team at Cleveland Clinic is able to assess prognosis, gauge potential treatment efficacy and determine the most sensitive testing modalities in monitoring for disease change. The Center for Genetic Eye Diseases is preparing for imminent participation in two gene therapy trials, one studying achromatopsia and one studying X-linked retinitis pigmentosa.
With a dedicated team of retinal dystrophy experts, retinal surgeons and an experienced ophthalmic genetic counselor, the Center for Genetic Eye Diseases continues to push the limits on novel ways to diagnose and treat patients with IRDs.
DeBenedictis is a clinical genetic counselor and research coordinator at Cleveland Clinic’s Cole Eye Institute. Dr. Traboulsi is Director of the Center for Genetic Eye Diseases at Cleveland Clinic’s Cole Eye Institute.
Advertisement
Advertisement
Advertisement

Motion-tracking Brillouin microscopy pinpoints corneal weakness in the anterior stroma

Registry data highlight visual gains in patients with legal blindness

Prescribing eye drops is complicated by unknown risk of fetotoxicity and lack of clinical evidence

A look at emerging technology shaping retina surgery

A primer on MIGS methods and devices

7 keys to success for comprehensive ophthalmologists

Study is first to show reduction in autoimmune disease with the common diabetes and obesity drugs

Treatment options range from tetracycline injections to fat repositioning and cheek lift