Latest results confirm and build on 16-week findings
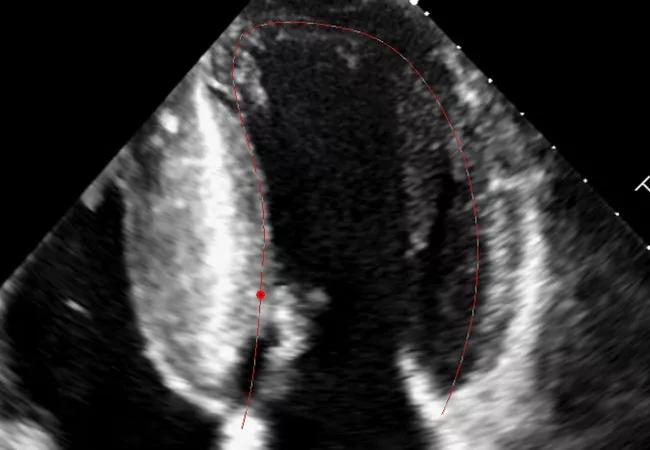
Mavacamten’s effects in reducing the need for septal reduction therapy (SRT) in patients with highly symptomatic obstructive hypertrophic cardiomyopathy (HCM) persist through 32 weeks of treatment, according to the latest data from the multicenter VALOR-HCM trial.
Advertisement
Cleveland Clinic is a non-profit academic medical center. Advertising on our site helps support our mission. We do not endorse non-Cleveland Clinic products or services. Policy
Trial participants initially randomized to mavacamten showed sustained reduction in the percentage proceeding to SRT or remaining guideline-eligible for SRT, and those initially randomized to placebo for the study’s first 16 weeks showed significant reductions in eligibility for SRT after being crossed over to mavacamten for weeks 16 to 32.
The 32-week results of the double-blind, randomized, placebo-controlled crossover trial were presented Nov. 6 in a featured science session at the at the American Heart Association Scientific Sessions by VALOR-HCM principal investigator Milind Desai, MD, MBA, Director of the Hypertrophic Cardiomyopathy Center at Cleveland Clinic. The study was simultaneously published online in Circulation.
“These latest results show that patients were able to safely remain on mavacamten for an extended period and collectively showed continued improvement, with even fewer meeting guideline criteria for septal reduction therapy at 32 weeks compared with 16 weeks,” says Dr. Desai. “These data provide further support for mavacamten’s potential to address an unmet need for noninvasive treatment of severe symptomatic obstructive HCM. We also observed important favorable changes in terms of cardiac remodeling that could have long-term implications in management of these patients.”
Mavacamten (Camzyos®) was approved by the FDA in April 2022 for treatment of adults with symptomatic obstructive HCM based on 16-week results of the VALOR-HCM trial, which Dr. Desai presented at the American College of Cardiology Scientific Session earlier this year.
Advertisement
No medication had previously been approved for this indication. While beta-blockers, calcium channel blockers and/or disopyramide may improve symptoms in patients with moderate left ventricular outflow tract (LVOT) obstruction, these drugs are typically inadequate when patients progress to severe symptomatic obstructive HCM. In such cases, SRT — surgical myectomy or alcohol septal ablation — is recommended and has been shown to improve long-term survival and quality of life.
Mavacamten is a targeted inhibitor of cardiac myosin designed to reduce the excessive contractility characteristic of obstructive HCM. It had previously been shown to improve LVOT gradient, quality of life and physical function in patients with obstructive HCM. “We undertook VALOR-HCM to determine mavacamten’s potential to serve as an alternative to septal reduction therapy in patients with severely symptomatic and refractory obstructive HCM,” Dr. Desai explains.
The study involved 112 enrollees at 19 U.S. sites, including Cleveland Clinic. All had documented HCM with maximum septal wall thickness of ≥ 15 mm (≥ 13 mm in those with a family history of HCM). Other key inclusion criteria were:
Advertisement
Patients could choose to proceed to SRT at any time.
Enrollees initially were randomized 1:1 to mavacamten 5 mg or placebo once daily by mouth. Randomization was stratified by type of SRT recommended (surgical myectomy or alcohol ablation) and by NYHA class. After assessment at the initial prespecified 16-week endpoint, the original mavacamten group continued the drug for 32 weeks and the placebo group was crossed over to dose-blinded mavacamten for weeks 16 to 32.
Mavacamten dosage was titrated throughout the study based on echocardiographic assessment of LVOT and LVEF every four weeks. The 5-mg daily dose could be reduced to 2.5 mg or increased up to 10 mg or eventually to 15 mg. Echo assessments were done by the study’s core lab at Cleveland Clinic.
The primary endpoint was a composite of the patient’s decision to proceed with SRT by week 32 or continued eligibility for SRT (according to 2011 ACC/AHA guidelines) at week 32. Patients and study staff were blinded to patients’ original assignment, mavacamten dose and echo data until week 32.
The study was coordinated by the Cleveland Clinic Coordinating Center for Clinical Research (C5Research) and Medpace (Cincinnati, Ohio).
As reported previously (and detailed at J Am Coll Cardiol. 2022;80:95-108), results at 16 weeks showed a significant reduction in the proportion of patients meeting SRT guideline criteria in the mavacamten group versus the placebo group.
Of the study’s 112 enrollees, 108 qualified for assessment at 32 weeks: 56 initially randomized to mavacamten and 52 initially randomized to placebo. The four patients excluded from the original placebo group included two who underwent SRT in the first 16 weeks and two who discontinued the study.
Advertisement
On the primary endpoint, results at week 32 were as follows:
The original mavacamten group showed a sustained reduction from baseline to week 32 in mean resting LVOT gradient (–33.0 mmHg) and mean provoked LVOT gradient (–43.0 mmHg), and similar mean reductions in LVOT gradients (–33.7 mmHg and –52.9 mmHg, respectively) were seen in the placebo crossover group after 16 weeks of mavacamten therapy.
“The reduction in the crossover group replicated the effects seen after 16 weeks of mavacamten therapy in the double-blind phase of the study,” Dr. Desai notes.
An improvement of at least 1 NYHA class was achieved by 90.6% of patients in the mavacamten group at 32 weeks and by 70% of patients in the placebo crossover group after 16 weeks of active therapy; additionally, mean Kansas City Cardiomyopathy Questionnaire score improved by 13.1 points in the original mavacamten group and by 8.0 points in the placebo crossover group.
These improvements were accompanied by favorable changes in cardiac remodeling as suggested by significant improvements in left ventricular mass index, left atrial volume index and markers of diastolic function in both groups.
Advertisement
There were no serious safety events, and no patients in the original mavacamten group met criteria for permanent discontinuation. Nine patients in the overall cohort temporarily discontinued therapy due to reduction of LVEF during mavacamten treatment; all remained asymptomatic, resumed treatment at a lower dose and continued in the study. “The study’s strategy of monthly echo monitoring to guide dosing and avoid excessive LVEF reduction due to mavacamten’s mechanism of action appears to be effective,” says Dr. Desai.
The investigators reported that 95% of patients had chosen not to undergo SRT and now remain in the study’s long-term extension phase. “This implies strong patient preference for a medical therapy option that may allow avoidance of an invasive procedure,” Dr. Desai observes.
“There is an encouraging similarity between the findings at 32 weeks in the crossover group and the 16-week findings in the mavacamten group from the initial analysis,” adds co-author Steven Nissen, MD, Chief Academic Officer of Cleveland Clinic’s Heart, Vascular & Thoracic Institute and chair of the VALOR-HCM executive steering committee. “These results show that patients are able to stay on mavacamten and improve sufficiently that they don’t need surgery or alcohol septal ablation. We will continue to follow them and assess whether that effect safely persists out to a year and beyond.”
The investigators note that the study carries significant potential implications for practice, in view of the limited number of high-volume centers for SRT and the association between SRT volumes and optimal outcomes.
“An effective noninvasive alternative to septal reduction therapy would provide a much-needed option for highly symptomatic patients and would expand our toolbox of offerings to these patients,” says VALOR-HCM co-author Nicholas Smedira, MD, MBA, Surgical Director of Cleveland Clinic’s Hypertrophic Cardiomyopathy Center. “Now that we have this evidence of improvement in symptoms with mavacamten, we are eager to learn whether long-term use of this agent reduces life-threatening arrhythmias and sudden death.”
Disclosure: The study was funded by MyoKardia, Inc., which markets mavacamten. Dr. Desai reports that he serves as a consultant for Myokardia.
Advertisement

End-of-treatment VALOR-HCM analyses reassure on use in women, suggest disease-modifying potential

Cardiac imaging substudy is the latest paper originating from the VANISH trial

Vigilance for symptom emergence matters, a large 20-year analysis reveals

Phase 3 ODYSSEY-HCM trial of mavacamten leaves lingering questions about potential broader use

5% of flagged ECGs in real-world study were from patients with previously undiagnosed HCM

High composite score in myectomy specimens signals worse prognosis
Few patients report left ventricular dysfunction or heart failure after one year
Avoidance of septal reduction therapy continues while LVEF dysfunction remains infrequent