Essentials of the NIH study and how it compares with REPAIR MR
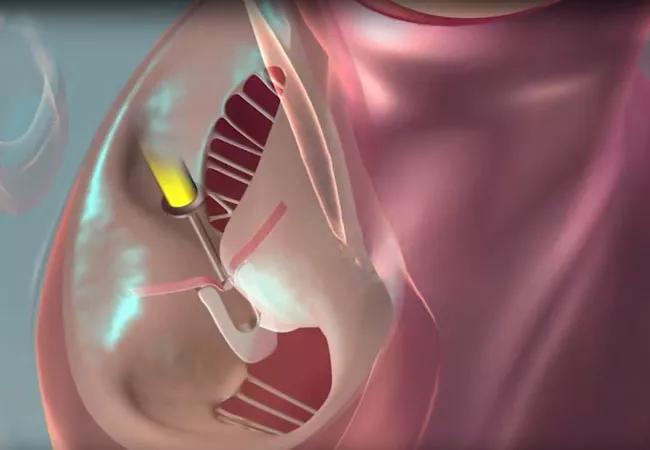
As the use of MitraClip™ for transcatheter edge-to-edge repair (TEER) of the mitral valve expands, the demand for data comparing this percutaneous procedure to surgical mitral valve repair is growing in tandem. In response, the National Institutes of Health (NIH) is funding the multicenter PRIMARY trial (NCT05051033) to generate such data and offer better guidance for patients.
Advertisement
Cleveland Clinic is a non-profit academic medical center. Advertising on our site helps support our mission. We do not endorse non-Cleveland Clinic products or services. Policy
The prospective, open-label, randomized study is comparing TEER with surgical repair in 450 patients aged 65 or older with primary degenerative mitral regurgitation (MR) and no other requirements for cardiac surgery. The study launched in January 2022 and will enroll patients at 20 or more centers in the U.S., Canada, Germany and the United Kingdom — including Cleveland Clinic.
“There is growing interest in the use of MitraClip in patients with degenerative MR (MR caused by prolapse), but we don’t yet have an evidence base to tell us in which patients MitraClip is a good option,” says cardiothoracic surgeon Marc Gillinov, MD, who is a leader of the trial at Cleveland Clinic and Chair of the NIH-funded Cardiothoracic Surgical Trials Network, which runs the trial. He notes that the issue is especially pressing in some European countries, such as Germany, where MitraClip is now being used more often than surgery for mitral valve repair.
“We generally hypothesize that surgery — even robotically assisted surgery — will create a more complete repair, but it’s also more invasive,” says Dr. Gillinov, Chair of Thoracic and Cardiovascular Surgery at Cleveland Clinic. “The PRIMARY trial should help indicate which therapy is better in which patients. As a government-funded study, it is designed to answer that patient-centered clinical question.”
Enrollees in PRIMARY must have severe primary degenerative MR and be appropriate candidates for either surgical or percutaneous mitral valve repair. Because enrollees may be at any level of surgical risk (low, intermediate or high) and MitraClip is authorized for U.S. commercial use only in patients at prohibitive surgical risk, the trial is being conducted under an Investigational Device Exemption. MitraClip is the only TEER device currently approved for commercial use in the U.S.
Advertisement
The study’s primary endpoint is a composite of all-cause mortality, valve reintervention, hospitalization for heart failure, or onset of ≥ 3+ MR by transthoracic echo at three years after randomization. Secondary outcomes include quality-of-life measures as well as adequacy of MR correction, defined as < 2+ MR, at one year after randomization. Outcomes will be measured over five years, and all patients will be followed for up to 10 years for certain endpoints. Primary study completion is projected for January 2028.
The PRIMARY trial is notable in several ways, according to Samir Kapadia, MD, Chair of Cardiovascular Medicine at Cleveland Clinic, who is leading the study at Cleveland Clinic along with Dr. Gillinov. One distinction is its use of the adequacy of MR correction as a secondary endpoint, which Dr. Kapadia says is novel for a large trial of this type.
Other distinctions are the inclusion of patients at all levels of surgical risk, which could support broadening the applicability of MitraClip, and the study’s use of a superiority design to detect a difference between treatments rather than to establish noninferiority.
“To date, most trials comparing surgery with minimally invasive therapies have used a noninferiority design,” Dr. Kapadia explains. “To show that patients should undergo surgery rather than a percutaneous procedure, you have to design a study to show that surgery is actually superior. This aspect of PRIMARY’s design is notable.”
The superiority design is in fact a key distinction between PRIMARY and another ongoing randomized trial comparing MitraClip with surgical mitral valve repair — the MitraClip REPAIR MR Study (NCT04198870) sponsored by Abbott Medical Devices, which is using a noninferiority design. REPAIR MR also is limited to patients at intermediate surgical risk, whereas PRIMARY includes patients at all surgical risk levels.
Advertisement
While Cleveland Clinic is not participating in the REPAIR MR study, Drs. Kapadia and Gillinov say it will be a helpful complement to the PRIMARY trial.
“The two trials are asking slightly different questions, but each should be valuable,” says Dr. Kapadia.
“It’s always best to have more than one trial and more than one set of investigators,” adds Dr. Gillinov. “If you have two trials that confirm one another, you can feel much more confident that their results weren’t caused by chance.”
Advertisement
Advertisement

Scenarios where experience-based management nuance can matter most

Introducing Krishna Aragam, MD, head of new integrated clinical and research programs in cardiovascular genomics

How Cleveland Clinic is using and testing TMVR systems and approaches

NIH-funded comparative trial will complete enrollment soon

How Cleveland Clinic is helping shape the evolution of M-TEER for secondary and primary MR

Optimal management requires an experienced center

Safety and efficacy are comparable to open repair across 2,600+ cases at Cleveland Clinic

Why and how Cleveland Clinic achieves repair in 99% of patients