The cancer stem cell (CSC) model of tumorigenesis and therapeutic resistance is a fairly recent but important development in oncologic research. Though experimental evidence has shown that CSCs drive many advanced cancers, researchers do not yet fully understand how they evade the immune system.
Cleveland Clinic is a non-profit academic medical center. Advertising on our site helps support our mission. We do not endorse non-Cleveland Clinic products or services. Policy
The work of Justin Lathia, PhD, a stem-cell biologist at Cleveland Clinic’s Lerner Research Institute, brings us closer than ever to this understanding. With funds from multiple NIH grants, including two R01s, Case Comprehensive Cancer Center, American Cancer Society and VeloSano, Dr. Lathia and his team hope to exploit their lab’s findings to disrupt CSC-driven resistance and growth in various malignancies.
Dr. Lathia’s latest research focuses on how CSCs thrive in the glioblastoma (GB) tumor microenvironment. Marked by hypoxia, acidic stress and necrosis, this toxic environment, a byproduct of the rapid and uncontrolled proliferation of cancer cells, is hostile to most cell types — except CSCs.
This exception intrigued Dr. Lathia and his colleagues. What do CSCs have that other cells don’t? Their recent publication in Cell Stem Cell provides an answer.
“We saw that there were two concepts at odds with each other, “ Dr. Lathia says of this research funded by Blast Glioblastoma, B*CURED, VeloSano and the Sontag Foundation. “One concept is that one of the hallmarks of advanced cancers are areas of necrosis… where the tissue runs out of nutrients and is highly stressed and the cells just die, and they emit their contents into the extracellular space.
“The other concept is that cells have evolved mechanisms to sense such damage, so if there’s an injury and a bunch of cells die in a given organ, that organ has the ability, even before the immune system responds, to sense the damage and respond to it. So how do cancer stem cells continue to persist? That was the central question of the paper.”
Through a side-by-side comparison of CSC and non-CSC reactions in specific toll-like immune receptor (TLR) cultures, Dr. Lathia and his coauthors found that CSCs in GB have adapted to express a lower level of the innate TLR4, the receptor that activates an immune response. These diminished TLR4 levels allow the CSCs to persist and multiply, repopulating a tumor in a hostile microenvironment.
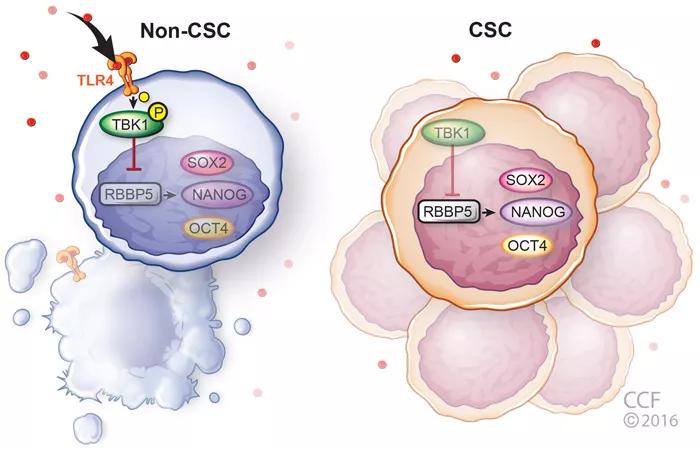
Figure. Model summarizing the role of TLR4 in glioblastoma. Non-CSCs possess TLR4 which can sense damage signals in the tumor microenvironment and suppress stem cell signaling via inhibition of RBBP5. CSCs, however, have low TLR4 expression which allows them to persist and expand despite damage signals in the tumor microenvironment. Figure republished with permission from Elsevier.
“It’s like CSCs have their headphones on and have lost ability to sense the environment around them,” says Dr. Lathia. “This is an evolutionary advantage that allows them to grow and persist, despite being damaged in the surrounding microenvironment.
“What really jumped out at us was if you put TLR4 ligands on cancer cells that are non-CSCs, they die. They really don’t like being cultured with TLR4 ligands, but the CSCs just don’t care,” Dr. Lathia says. “You can put any ligand you want on a cell, and the cell is only going to respond if it has the corresponding receptor. We screened all toll-like receptors and found that CSCs had a very low level of TLR4.” The team then tested their findings by engineering CSCs with a higher level of TLR4. The result? The CSCs grew more slowly and lost their stem-cell characteristics.
Knowing that it would be difficult to restore TL4R receptors to CSCs, Dr. Lathia’s team looked downstream of the protein and found the transcription factor retinoblastoma binding protein 5 (RBBP5), which has an inverse relationship with TLR4. Using RNA interference, they reduced the levels of RBBP5 in GB cells and found the CSCs didn’t grow or self-renew as well. They also discovered that RBBP5 is part of the epigenetic complex that ensures CSC transcription factors remain activated. Thus if RBBP5 is suppressed, Dr. Lathia reasoned, CSCs might not replicate.
“We were basically able to map out a signaling pathway that starts with TLR4 and involves RBBP5 and the core stem cell circuitry,” he says. “The reason this is important is this is one of the first examples of an immune signaling response directly interacting with the stem cell circuitry.”
The therapeutic answer might seem obvious — inject GB patients’ tumors with TLR4. In fact, this type of approach is being used in some clinical trials involving other toll-like receptors and other types of cancers. Dr. Lathia, however, has concerns. He hypothesizes that such an approach might change the dynamics of the tumor, killing all the non-CSC cancer cells but leaving behind a plethora of CSCs. His lab is currently investigating this approach, adding TLR4 ligands to GB cells in preclinical mouse models to see if such therapies could be a long-term solution for patients.
In addition to their work on TLR4, Dr. Lathia’s team is exploring the questions of CSC-driven tumor progression and therapeutic resistance from many angles. One is cell-to-cell communication, specifically a class of cell surface channels called connexins that form gap junctions and directly connect the cytoplasm of the two cells. Gap junctions can rapidly and efficiently pass cargo — molecules, ions and electrical impulses — between cells. The team has shown that specific connexin channels in GB and breast cancer can accelerate tumor growth. They are now researching the signaling process to understand how that happens and have also leveraged this work to include prostate cancer and leukemia.
His lab is also using high-throughput screening to sift through more than 800 FDA-approved compounds to find those that might inhibit this particular cell-to-cell communication. They have found 10 and, so far, have shown one will extend survival in tumor-bearing mice models. They plan to chemically modify these parent compounds to make new drugs that will inhibit cell-to-cell communication between malignant cells.
Dr. Lathia’s lab is also pursuing how to shift the balance in the immune system to better fight brain cancer. Certain cancers such as GB accumulate myeloid-derived suppressor cells (MDSCs) that suppress the immune system. In tumor cells, however, MDSCs can also suppress cytotoxic T cells, rendering them impotent.
To flip that scenario, Dr. Lathia and Cleveland Clinic colleagues David Peereboom, MD, medical oncologist, and Michael Vogelbaum, MD, PhD, Associate Director of Cleveland Clinic’s Rose Ella Burkhardt Brain Tumor and NeuroOncology Center, are conducting a phase 1 clinical trial that attempts to target MDSCs in recurrent GB patients using low-dose chemotherapy.
“We’re trying to inverse the immune suppression, to inhibit the inhibitors,” Dr. Lathia says. “We’ve tried it in myriad ways, but one blunt way is just by giving patients low dose chemotherapy such as 5-fluorouracil, which has been around for decades. If you give it twentyfold less than traditionally used for a cancer patient, you can actually target the MDSCs.”
The low dose, which exploits MDSCs’ relative sensitivity to chemotherapy, safeguards the cytotoxic T cells. “In a 2016 paper in Stem Cell, we showed that if you increase the dose of 5-fluorouracil high enough you can kill cytotoxic T cells as well, which we don’t want to do. But there’s a sweet spot, a therapeutic window that we think we have found.”
Dr. Lathia’s lab has active projects in cell adhesion mechanisms, cell-cell communication and the interaction between tumor cells and the immune system. “Our next step is developing new methods to track the stem-cell state in real time and single-cell cell-fate decisions,” says Dr. Lathia. “This should bring us closer to fully understanding and thus being able to disrupt the process.”
Dr. Lathia is Associate Staff in the Department of Cell and Molecular Medicine and Cleveland Clinic’s Rose Ella Burkhardt Brain Tumor and NeuroOncology Center. On Twitter: @JustinLathia
Photo Credit: ©Russell Lee

First-of-its-kind research investigates the viability of standard screening to reduce the burden of late-stage cancer diagnoses

Study demonstrates ability to reduce patients’ reliance on phlebotomies to stabilize hematocrit levels

Findings highlight an association between obesity and an increased incidence of moderate-severe disease

Cleveland Clinic Cancer Institute takes multi-faceted approach to increasing clinical trial access

Key learnings from DESTINY trials

Gene editing technology offers promise for treating multiple myeloma and other hematologic malignancies, as well as solid tumors

Study of 401,576 patients reveals differences in cancer burdens as well as overall survival

Enfortumab plus pembrolizumab reduced risk of death by 53% compared with platinum-based chemotherapy