TVT Registry analysis could expand indication to lower surgical risk levels
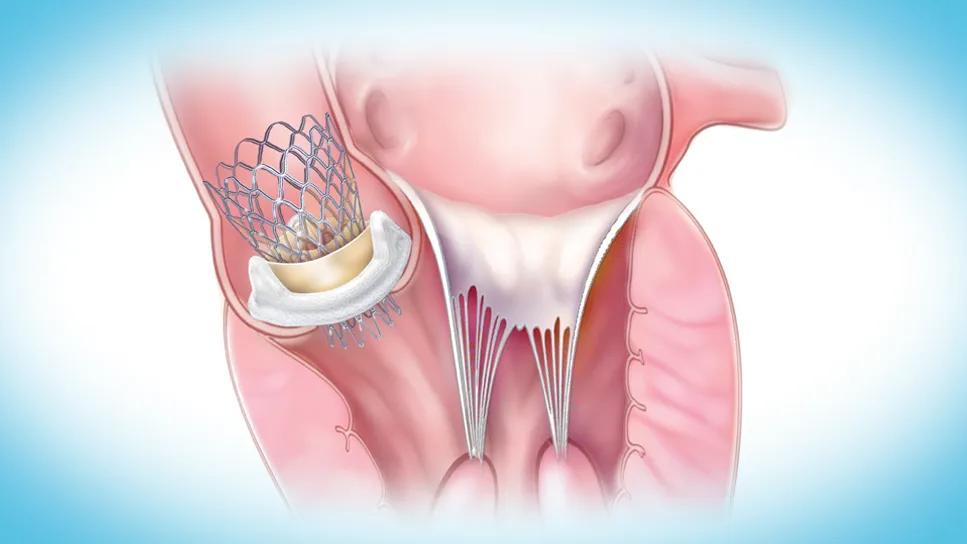
Five-year outcomes of valve-in-valve transcatheter aortic valve replacement (ViV TAVR) for patients with degenerated surgical valves showed the procedure to be as safe as native valve TAVR in patients at all levels of surgical risk. So finds a new analysis of the Society for Thoracic Surgeons (STS)/American College of Cardiology TVT Registry published in in JACC: Cardiovascular Interventions (2025;18[16]:1989-2000).
Advertisement
Cleveland Clinic is a non-profit academic medical center. Advertising on our site helps support our mission. We do not endorse non-Cleveland Clinic products or services. Policy
“The findings confirm what we were expecting and hoping for, which is that ViV TAVR, as it pertains to balloon-expandable platforms, demonstrates excellent safety and durability at all surgical risk levels,” says Amar Krishnaswamy, MD, Section Head of Interventional Cardiology at Cleveland Clinic and the study’s first and corresponding author.
While the TVT Registry shows that the frequency of ViV TAVR rose significantly from 410 cases in 2014 to 4,480 in 2019, clinical outcomes data in the TVT Registry are limited to one-year follow-up. Dr. Krishnaswamy and colleagues felt that mid-term results were needed to better ascertain outcomes in real-world use. “This is especially important in light of proposals to consider expanding the indication to patients at intermediate to low levels of surgical risk,” he says.
The multicenter study group conducted a TVT Registry-based analysis of all patients (N = 14,474) undergoing transfemoral ViV TAVR with a balloon-expandable valve from June 2015 to March 2023. To extend follow-up beyond the one year reported in the TVT Registry, the researchers used claims data from the Centers for Medicare and Medicaid Services (CMS) database. Death, stroke and reintervention were tracked for up to five years.
The 14,474 ViV TAVR patients were propensity-matched with 385,556 patients who underwent native valve TAVR in the same period, resulting in 13,638 matched pairs.
Patients were grouped by baseline STS Predicted Risk of Mortality (PROM) score. Within the propensity-matched ViV TAVR cohort (mean age, 74 years), the mean STS PROM score was 6.1; 5,952 patients were at low surgical risk, 4,724 at intermediate risk and 3,155 at high risk.
Advertisement
At five years, the ViV TAVR group showed lower levels of the primary endpoints of death (43.1% vs. 55.2%), stroke (10.5% vs. 11.8%) and their composite relative to native valve TAVR (P < .001 for all). Findings were similar at each of the three levels of STS PROM risk scores.
Despite superior freedom from death and stroke in the the ViV TAVR group, reintervention rates for valve failure at five years — which were relatively low overall — were higher for ViV TAVR than for native valve TAVR (4.7% vs. 1.8%; P < .001). Stenosis was the most common cause for reintervention in the ViV group.
“This is important for two reasons relating to the index surgical and index valve-in-valve procedures,” Dr. Krishnaswamy notes. “We need to make sure surgeons place valves that are large enough to accommodate an appropriately sized valve-in-valve TAVR prosthesis and allow for its full expansion. That may require root enlargement or other techniques in the index surgery.
“It’s also relevant to the valve-in-valve procedure that we appropriately expand TAVR valves,” he continues, “which have the propensity to be underexpanded when we put them into a stiff surgical frame. Adjuvant procedures, such as bioprosthetic valve modification or bioprosthetic valve fracture, allow the valve to be expanded to its fullest dimension. This is important for optimizing hemodynamics and reducing risk of leaflet thrombus. I believe the more common need for reintervention in the valve-in-valve group implies, at least to some degree, that lack of full expansion is more common in valve-in-valve procedures than in native valve TAVR.”
Advertisement
The number of patients with surgical valve degeneration continues to grow, and increasing numbers of younger patients are electing bioprosthetic surgical AVR (SAVR). Both factors will lead to increasing need for the treatment of bioprosthetic valve failure, Dr. Krishnaswamy notes.
“If you assume a surgical valve will last 10 years, younger patients will be coming back for a valve-in-valve TAVR or redo surgery,” he says. “We need to determine how to arrange treatment options to minimize the open-heart surgeries a patient will need during their lifetime. Broadening the indication for valve-in-valve TAVR beyond patients at high surgical risk would allow us to provide a less-invasive option to a larger number of patients, as we have seen in the setting of native aortic valve stenosis.”
“While valve-in-valve TAVR is attractive due to its minimally invasive nature with reduced potential for periprocedural complications, studies comparing the long-term outcomes of redo SAVR versus valve in valve TAVR out to 10 years are needed,” adds Cleveland Clinic cardiothoracic surgeon James Yun, MD, PhD. “Such studies will eventually inform us whether redo SAVR is associated with less need for valve reintervention over the long term.”
At Cleveland Clinic, the same process used to assess suitability for native valve TAVR is followed when considering ViV TAVR in patients at low to intermediate surgical risk.
“Patients are assessed anatomically by the heart team for both TAVR and SAVR,” Dr. Krishnaswamy says. The heart team discussion about safety, feasibility and risk for each pathway is individualized for patients based on the following questions:
Advertisement
According to Dr. Krishnaswamy, ViV TAVR is at a point where another randomized study in each surgical risk group would be impractical.
“The size of this analysis and the rigorous detail we paid to assessing outcomes and length of follow-up should reassure physicians and patients about the safety and durability of valve-in-valve TAVR at all surgical risk levels up to five years,” he says.
“This is the largest series on the topic published to date,” he adds. “It may provide the necessary data, together with the smaller studies that have been published, to broaden the indication for valve-in-valve TAVR.”
Advertisement
Advertisement

Patient series and bench validation support efficacy and safety of CLEVE procedure

In the wake of NOTION-3 findings, a strong argument for physician judgment remains
Post hoc analysis of PROTECTED TAVR finds reduced stroke risk in the U.S. but not beyond
Analysis of STS/ACC TVT Registry finds greatest benefit in patients with prior stroke

TAVR explant demands multidisciplinary expertise

How our HVTI Advisory Services team facilitated swift improvements for an allied health organization
Support for a TAVR-first approach in patients with concurrent valve and coronary disease
Five-year data demonstrate convergence of outcomes from years 1 to 5